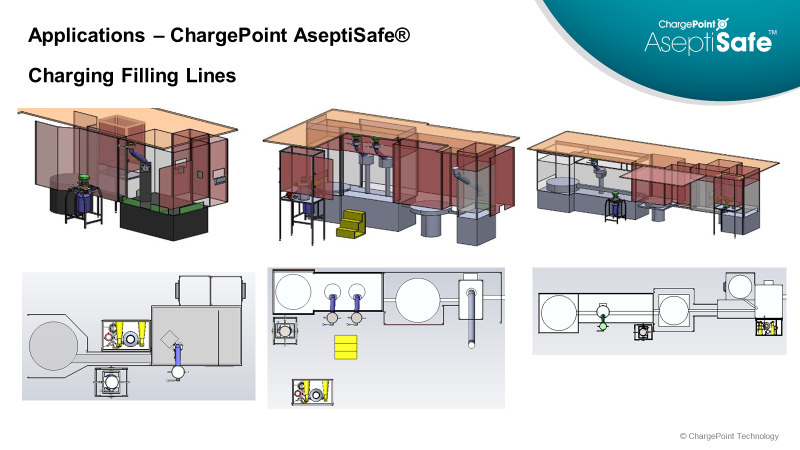
ChargePoint’s aseptic powder transfer valves offer increased sterility assurance when handling sensitive ingredients and small components in fill/finish aseptic processing and biotech sterile API production.
The ChargePoint AseptiSafe® range provides multiple benefits to the user and the product process including
- Maintaining critical area integrity
- Removing the need for high air class control areas and cumbersome PPE
- Toxic powder processing whilst ensuring the safety of your personnel and a dust free environment
- Maximising yield transfer for poorly flowing and high value product
- Several alternative methods of sterilising the ChargePoint AseptiSafe® valve contact and sealing faces are available to meet the critical area and process set-up requirements of our customers.
ChargePoint AseptiSafe®
- Sterilisation method: autoclave or/and SIP (steam in place)
- Offers a simple upgrade to GMP requirements in existing or new facilities
- SIP prior to material transfer
- Ideal for installation in higher grade cleanrooms with Isolator/RABS or existing LAF or other higher air class control systems
ChargePoint AseptiSafe® pro
- Sterilisation method: SIP/ISO5 HEPA LAF
- In-board ports direct clean Grade A air directly across the valve interface
- Remote filter/fan unit supplies air to the terminal HEPA filter positioned next to the valve interface
- Can be used for high containment dust control with dust particulate collected from the second port and returned to air filtration in a closed loop system